WAITING ON YOUR FIRST-IN-HUMAN (FIH) MATERIAL?
Don't Let Time Hold You Back.
Traditional drug development presents ample challenges in cell target screening and candidate selection, including the inherent risks and delays of process development and tech transfers. That’s why we offer a truly integrated, end-to-end solution. Whether you have a fully confirmed lead, a pool of binders we can optimize, or are just starting your drug development journey, Resilience can get your innovation into the clinic faster.
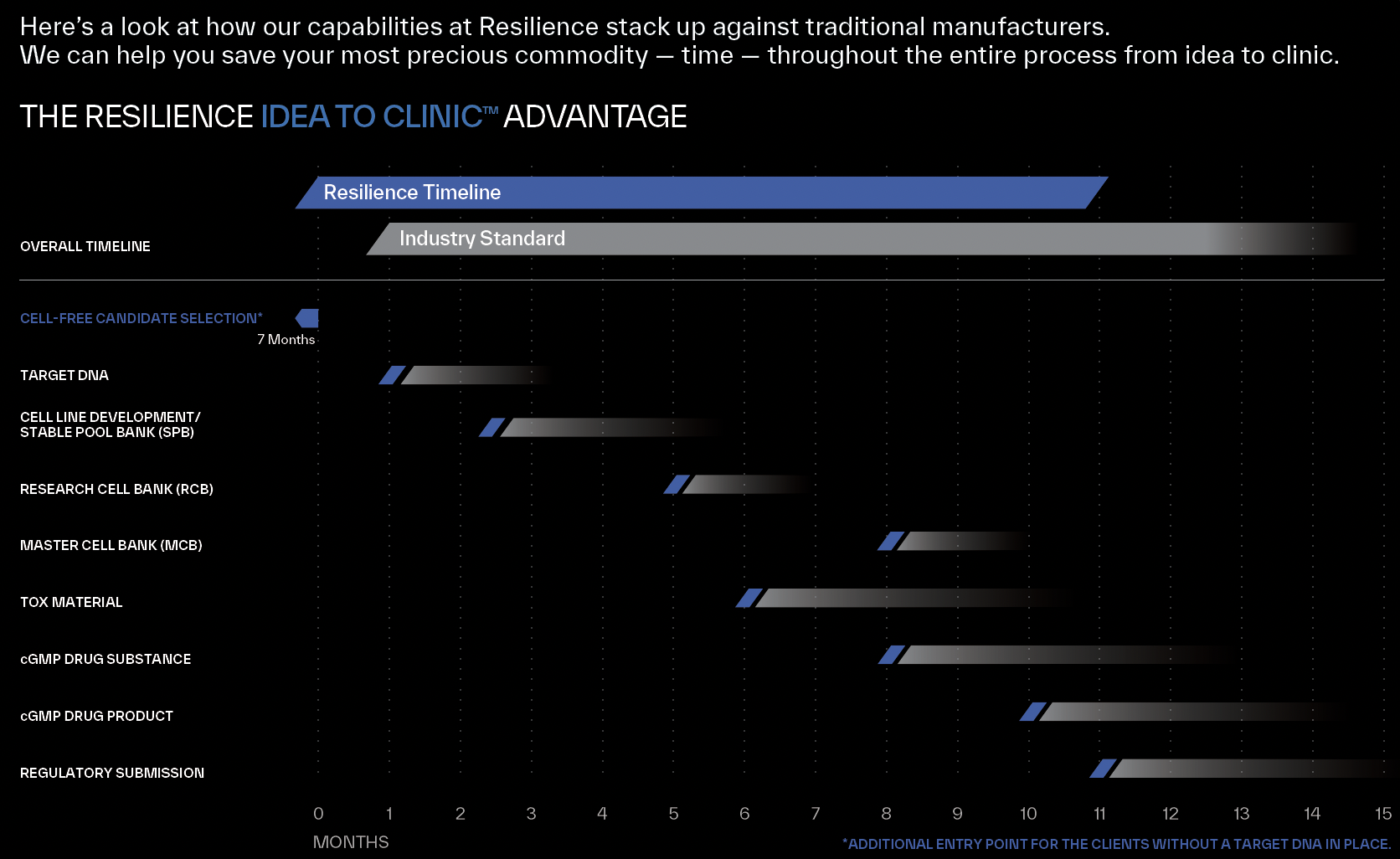
OUR AIM: TO DELIVER A ~30% TIME SAVINGS FROM START TO FINISH
Getting you from ideation to clinic in record time demands a simultaneous focus on big data and small details. That’s why at Resilience, we’re combining cutting-edge molecular development techniques using cell-free synthesis and machine learning-based protein design to accelerate and de-risk your therapeutic timeline. We couple that with our high-productivity and reliable manufacturing platform to streamline the path from DNA sequence to GMP drug substance and drug product manufacturing processes through to your regulatory filing.
A one-stop shop that builds therapeutics right at the start
While most CDMOs typically engage clients from cell line development to drug product, Resilience offers comprehensive support that begins as early as the lead stage and extends through to regulatory submission.
Preserving your most precious asset: time
With an aim to reduce your timelines by approximately 30% compared to industry standards, we can expand your scope to create greater program longevity.
Unlocking Flexibility: Embrace Multiple Entry Points
Discovery stage: Resilience speeds up candidate selection by over four months using our innovative cell-free platform. With our services, you can seamlessly transition to us as your Phase 1 material provider.
Pre-clinical: Resilience accelerates your manufacturing timeline for First-In-Human materials with our expertise in process development and stable CHO pool technologies.
Future-proof with our regulatory expertise
Our in-house regulatory services allow clients to consolidate all First-In-Human manufacturing needs under one roof, eliminating the need for expensive third-party CMC consultancy services.